

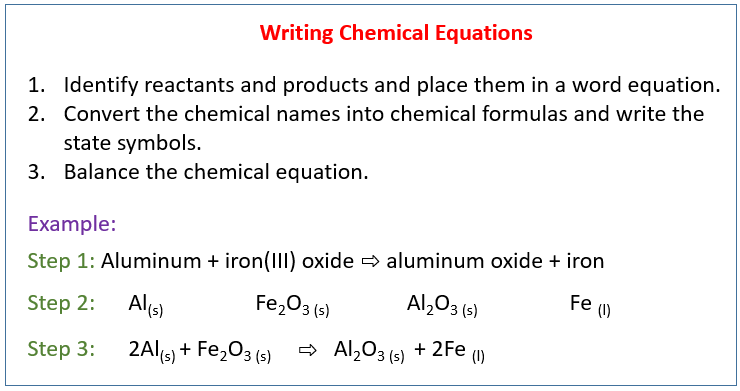
Instructions on balancing chemical equations: Please tell about this free chemistry software to your friends! We use an algebraic equation to relate the rate of reaction, -r A, to the concentration of reacting species (e.g., C A) and to the temperature (T) at which the reaction occurs. r j is an algebraic equation, not a differential equation.r j is independent of the type of reaction system (batch, plug flow, etc.).r j is a function of concentration, temperature, pressure, and the type of catalyst (if any).r j is the rate of formation of species j per unit volume.NOTE: dC A/dt is not the rate of reaction Which is the rate of disappearance of species A on a per mass of This also means that the rate of formation of A is r A = -0.2 mole/dm 3/s.įor a catalytic reaction, we refer to -r A ', Then A is disappearing at the same rate (-r A = 0.2 mole/dm 3/s). If B is being created at a rate of 0.2 moles per decimeter cubed per second (i.e. R B = the rate of formation of species B per unit volume r A = the rate of a disappearance of species A per unit volume R A = the rate of formation of species A per unit volume The rate of a reaction can be expressed as the rate of disappearance of a reactant or as the rate of appearance of a product. The reaction rate is the rate at which a species looses its chemical identity per unit volume.
Please try using a different browser or updating your current browser. Sorry, your browser does not support the audio tag.

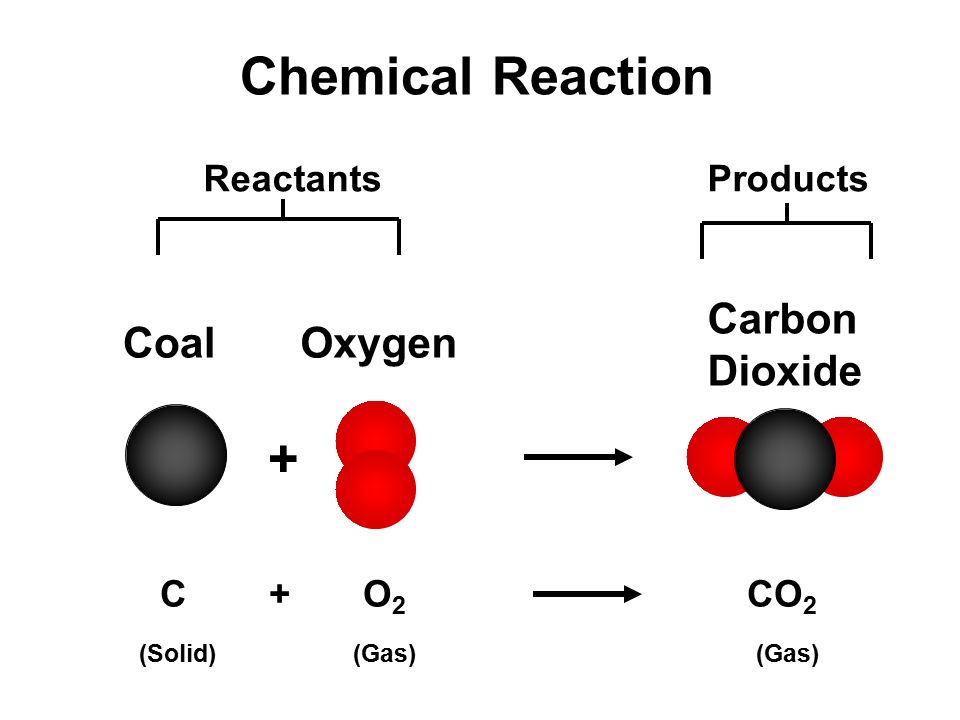
Three ways a chemical species can lose its chemical identity: 1. Reaction YouTube Video: Carbon Dioxide and Magnesium


 0 kommentar(er)
0 kommentar(er)
